
#Molar mass finder manual
References:įrom the source of Wikipedia : Basic definition & example.įrom the site of : Manual calculation of the simplest ratio formula.įrom the authorized source of ACS : The formulas of different compounds.
So, keep the empirical formula calculator to find the simplest whole-number ratio as well as the empirical formula of the compound with a step-by-step solution. Having an idea of what reaction is going to produce ensures that the reactions safer and more efficient to the consumer of the reaction. When it comes to real-world chemistry, it becomes even trickier since atoms sometimes participate in unusual bonds, thus, these formulas aren’t necessarily accurate. The Density Calculator uses the formula pm/V, or density (p) is equal to mass.
#Molar mass finder free
The technique for determining the simplest ratio formula can be considered in almost any real-world solution. Free online physics calculators, velocity equations and density, mass and. test tube burette funnel corks tubes calculator. That’s the reason why it’s is also said to be the simplest ratio. The molar mass of a substance is easy to determine: simply add up the molar masses of the. Remember that the empirical formula is referred to as the smallest whole-number ratio. A simple, but actual example of this concept is that the empirical formula of sulfur monoxide would simply \(SO\) as it is derived from formula disulfur dioxide, \(S_H_4)_n\). You can also try our online mole fraction calculator that helps you to calculate the mole fraction, moles of solute, moles of solvent according to the given input parameters.Īccording to chemistry definition, the empirical formula of a chemical compound is referred to as the simplest positive integer ratio of atoms present in a compound.
#Molar mass finder how to
Just read on the context to understand how to find the empirical formula, its basic definition in chemistry, and much more! This combustion analysis calculator considers the symbol & percentage mass of the element & determine the simplest whole-number ratio of atoms in a compound. The National Institute of Standards and Technology published the atomic weights for each element and is a good resource to consult.An online empirical formula calculator allows you to find empirical formula corresponding to the given chemical composition. You can calculate the molecular weight of the compound by adding the atomic masses of each element in the compound using the periodic table. Each of these steps uses the molar mass as a conversion factor between grams and moles. Then the product of the reaction is weighed to calculate the yield of the reaction in grams, which can be converted to moles. The reactants must be weighed out in the correct amounts based on the number of moles required. However, the reactants and products are frequently measured in grams or liters. Chemical reactions are normally described in terms of moles of reactants and moles of products. Molar mass is used to convert between the weight of a substance and the number of moles of that substance in chemistry.

For most practical purposes, a Dalton can be considered equivalent to g/mol. It is also sometimes called: Molecular Mass, Molecular Weight, Formula Mass, or Formula Weight.
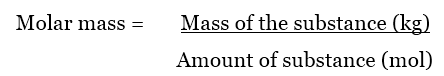
One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. A Dalton is similar to g/mol but is used for more precise measurements of atoms and subatomic particles in chemistry and physics. Molar mass is the mass (in atomic mass units) of one mole of a of a substance. This numerical value is known as Avogadro’s number.Īnother unit often used to describe molecular weight or molar mass is the Dalton (Da). One mole is equal to 6.02214076 × 10 23 elementary entities, such as atoms, molecules, electrons, or ions. The atomic mass is the mass of 1 mol of an atom – so if you add up the atomic masses of all the atoms in the compound, the sum is the molar mass or molecular weight of that compound expressed in g/mol.Ī mole is used as a standard measurement for a very large quantity of very small units of matter. Molar mass, sometimes referred to as molecular weight or molecular mass, is equal to the sum of the atomic masses of each element in the compound. Molar mass is the mass of a compound in grams per mole of a substance, and it’s expressed in g/mol.


 0 kommentar(er)
0 kommentar(er)
